Free digital trial for your classroom
Set your classroom up with an online trial of Chemistry for VCE for a full school term and explore the market-leading suite of digital resources.*
Sign up for a free trial and your local Education Consultant will work with you to ensure a seamless transition.
*Only available to practicing secondary school teachers.

We have you covered for the 2023–2027 VCAA Study Design
- Units 1 & 2 available for implementation in 2023
- Units 3 & 4 available for implementation in 2024
Key features of the new Study Design, including Sustainability and Scientific Methodologies, are also explicitly targeted in this new series.
Streamlined to the Study Design
Forget irrelevant content, this brand new series provides more opportunities to apply and extend understanding of Key Knowledge and Skills
Supporting every step to assessment success
Set your students on a pathway to assessment success and provide targeted support for every step along the way: Schools Assessed Coursework (SACs), practical work and exams.
40+ hours of practical work?
No problem!
Targeted instructional videos, risk assessments and lab tech notes will provide the support you need to complete over 10 hours of practical work per unit as required by the new Study Design.
Chemistry for VCE Units 1 & 2
View Table of contents View full draft Student Book Request Digital SampleChemistry for VCE Units 1 & 2
Student Book + obook pro
ISBN: 9780190337933
RRP: $84.95
Student Book + obook pro
Buy now Learn moreStudent Book + obook pro ?Print Student Book with digital obook pro access for 27 months from the date of activation
Chemistry for VCE Units 1 & 2
Student obook pro
ISBN: 9780190337940
RRP: $64.95
Student obook pro
Buy now Learn moreStudent obook pro ?Digital Student obook pro access for 27 months from the date of activation
Chemistry for VCE Units 1 & 2
Teacher obook pro
Teacher digital access available to adopting schools
Teacher obook pro ?Free Teacher obooks only available to schools adopting our series with Student books. Speak to your local education consultant for an annual subscription.
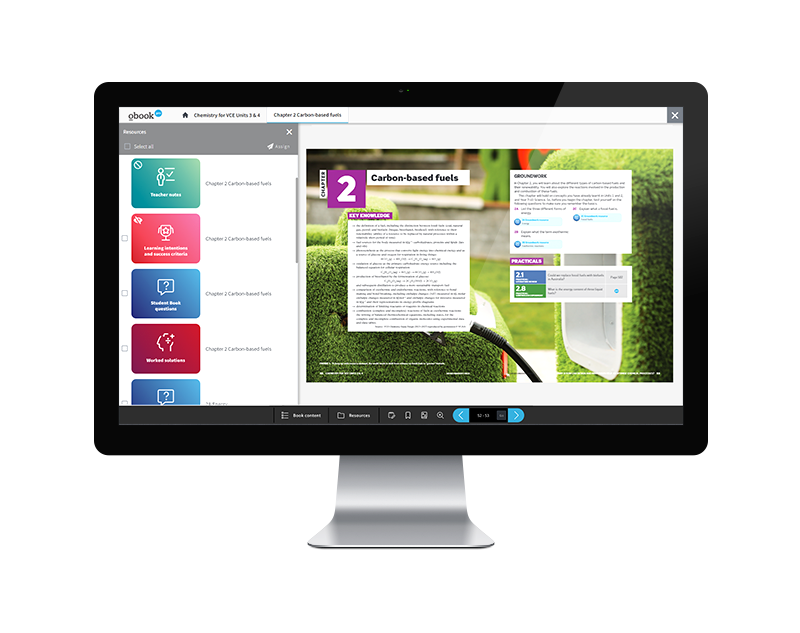
Chemistry for VCE Units 3 & 4
View Table of contents View full draft Student Book Request Digital SampleChemistry for VCE Units 3 & 4
Student Book + obook pro
ISBN: 9780190337971
RRP: $84.95
Student Book + obook pro
Buy now Learn moreStudent Book + obook pro ?Print Student Book with digital obook pro access for 27 months from the date of activation
Chemistry for VCE Units 3 & 4
Student obook pro
ISBN: 9780190337988
RRP: $64.95
Student obook pro
Buy now Learn moreStudent obook pro ?Digital Student obook pro access for 27 months from the date of activation
Chemistry for VCE Units 3 & 4
Teacher obook pro
Teacher digital access available to adopting schools
Teacher obook pro ?Free Teacher oonly available to schools adopting our series with Student books. Speak to your local education consultant for an annual subscription.
Changes to the VCAA Chemistry Study Design (2023-2027)
Changes to the VCAA Chemistry Study Design (2023-2027) affect all aspects of teaching and learning. New key knowledge, course structure, scientific methodologies and a fresh approach to assessment.
What's in, what's out? Allow our expert publishers to guide you through the content changes and help to confidently implement the VCAA Chemistry Study Design.
The 2023 VCE Chemistry Study Design also includes:
- More guidance and definitions for key terms relating to data and measurement (pages 18–19)
- More inclusion of Aboriginal and Torres Strait Islander cultures and knowledges
- Refinements to Key Science Skills (e.g. now refers to data generation instead of data collection) (pages 11–12)




